- HOME
- »
- BIOSIMILARS
BIOSIMILARS
![]()
![]()
![]()
![]()
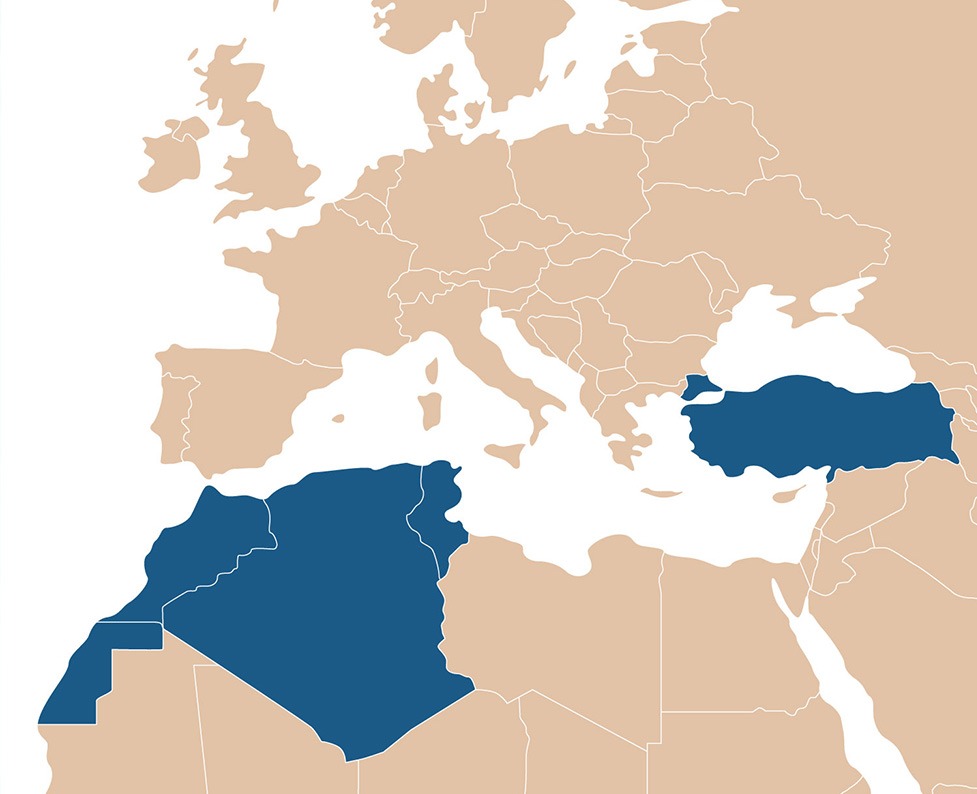
Some countries as Turkey, Algeria, Moroco, Tunisia, biosimilars of Orphan Drugs could be introduced earlier than in the rest of Europe, as there is no EU or US exclusivity. Those countries are representing almost 200M inhabitants all together. Do not miss these opportunities!
Biosimilars are copies of approved innovator biotherapeutic products that have been demonstrated to be comparable to the corresponding original product in terms of quality, safety and efficacy. They offer improved affordability since they come on the market at a significantly reduced price compared to the original product.
For Orphan Drugs, despite the specific « enlarged » patent protection given by Orphan Drug status, biosimilars constitute the beginning of a brand new era, by fostering access to all patients in need, especially in emerging countries.
From a regulatory perspective, biosimilars, being complex molecules, are subject to more stringent criteria for approval compared to generic products. Analytical and clinical comparability in terms of quality, safety and efficacy with comparison to the original product, should be established to secure access to the market.
BIOSIMILARS
![]()
![]()
![]()
![]()
Some countries as Turkey, Algeria, Moroco, Tunisia, biosimilars of Orphan Drugs could be introduced earlier than in the rest of Europe, as there is no EU or US exclusivity. Those countries are representing almost 200M inhabitants all together. Do not miss these opportunities!
Biosimilars are copies of approved innovator biotherapeutic products that have been demonstrated to be comparable to the corresponding original product in terms of quality, safety and efficacy. They offer improved affordability since they come on the market at a significantly reduced price compared to the original product.
For Orphan Drugs, despite the specific « enlarged » patent protection given by Orphan Drug status, biosimilars constitute the beginning of a brand new era, by fostering access to all patients in need, especially in emerging countries.
From a regulatory perspective, biosimilars, being complex molecules, are subject to more stringent criteria for approval compared to generic products. Analytical and clinical comparability in terms of quality, safety and efficacy with comparison to the original product, should be established to secure access to the market.
While countries in North Africa like Algeria, Tunisia and Morocco have their own laws/regulations for the registration of new pharmaceutical products, they still have not yet established legal/regulatory frameworks for biosimilars. Still, these countries represent an important market with a population of over 82M inhabitants that could have access to biosimilars as long as the local regulations and process/pathways are respected.
The Algerian Drug Market is the second largest in the MENA region and the most solvent, mainly due to a very generous social protection system with broad health coverage and public health institutions. In Algeria, biosimilar legislation is expected to be introduced shortly. Meanwhile, the country had already seen biosimilars successfully registered in hematology, oncology and endocrinology, among others, relying in part on the WHO, EMA, and FDA recommendations and the advice of several expert committees of different specialties. Being registered in its country of origin is a major criterion, among others, to be able to apply for registration.

While countries in North Africa like Algeria, Tunisia and Morocco have their own laws/regulations for the registration of new pharmaceutical products, they still have not yet established legal/regulatory frameworks for biosimilars. Still, these countries represent an important market with a population of over 82M inhabitants that could have access to biosimilars as long as the local regulations and process/pathways are respected.
The Algerian Drug Market is the second largest in the MENA region and the most solvent, mainly due to a very generous social protection system with broad health coverage and public health institutions. In Algeria, biosimilar legislation is expected to be introduced shortly. Meanwhile, the country had already seen biosimilars successfully registered in hematology, oncology and endocrinology, among others, relying in part on the WHO, EMA, and FDA recommendations and the advice of several expert committees of different specialties. Being registered in its country of origin is a major criterion, among others, to be able to apply for registration.
Similarly, Tunisia applies international procedures for submission, evaluation and registration of biosimilars in addition to advice from specialized scientific commissions, among other parties. Several biosimilars for different diseases have been thus approved in Tunisia. The country recognizes the value of introducing biosimilars. Still, a strict legal and regulatory framework is yet to be established.
To ensure smooth access to the market, defining your product’s potential, knowledge of the different local pathways and efficient field operation are key success factors without which bureaucracy and complex procedures would represent serious hurdles.
CarthaGenetics® has the expertise dedicated to the introduction of orphan drugs biosimilars in emerging countries like Algeria and Tunisia. We are committed to help you manage successful biosimilar programs from registration to commercialization/tender – in the most cost-efficient way.
To know more about biosimilars’ regulatory and market access landscape in North Africa, please ask our experts at: biosimilar@carthagenetics.com. CarthaGenetics® offers the first 30 minutes consultancy session for free.

Similarly, Tunisia applies international procedures for submission, evaluation and registration of biosimilars in addition to advice from specialized scientific commissions, among other parties. Several biosimilars for different diseases have been thus approved in Tunisia. The country recognizes the value of introducing biosimilars. Still, a strict legal and regulatory framework is yet to be established.
To ensure smooth access to the market, defining your product’s potential, knowledge of the different local pathways and efficient field operation are key success factors without which bureaucracy and complex procedures would represent serious hurdles.
CarthaGenetics® has the expertise dedicated to the introduction of orphan drugs biosimilars in emerging countries like Algeria and Tunisia. We are committed to help you manage successful biosimilar programs from registration to commercialization/tender – in the most cost-efficient way.
To know more about biosimilars’ regulatory and market access landscape in North Africa, please ask our experts at: biosimilar@carthagenetics.com. CarthaGenetics® offers the first 30 minutes consultancy session for free.
