- HOME
- »
- PATIENT IDENTIFICATION

Patient identification and patients recruitment for your clinical trials
3 exemples of working with Carthagenetics®:
• For a rare lysosomal disorder, CarthaGenetics® has been able to obtain clinical approvals in 2 months in Turkey with a strong collaboration with the global CRO picked up by our customer.
• Due to its network, CarthaGenetics® found for another ultra rare disorder 58 patients in one country, where a big pharma with a competitive treatment and many sales reps in the field has only identified 5 !
• For another Ultra rare disorder, CarthaGenetics® helped some European patients relocation in the US for the duration of a phase III in the USA.
Patient identification and patients recruitment for your clinical trials
3 exemples of working with Carthagenetics®:
• For a rare lysosomal disorder, CarthaGenetics® has been able to obtain clinical approvals in 2 months in Turkey with a strong collaboration with the global CRO picked up by our customer.
• Due to its network, CarthaGenetics® found for another ultra rare disorder 58 patients in one country, where a big pharma with a competitive treatment and many sales reps in the field has only identified 5 !
• For another Ultra rare disorder, CarthaGenetics® helped some European patients relocation in the US for the duration of a phase III in the USA.
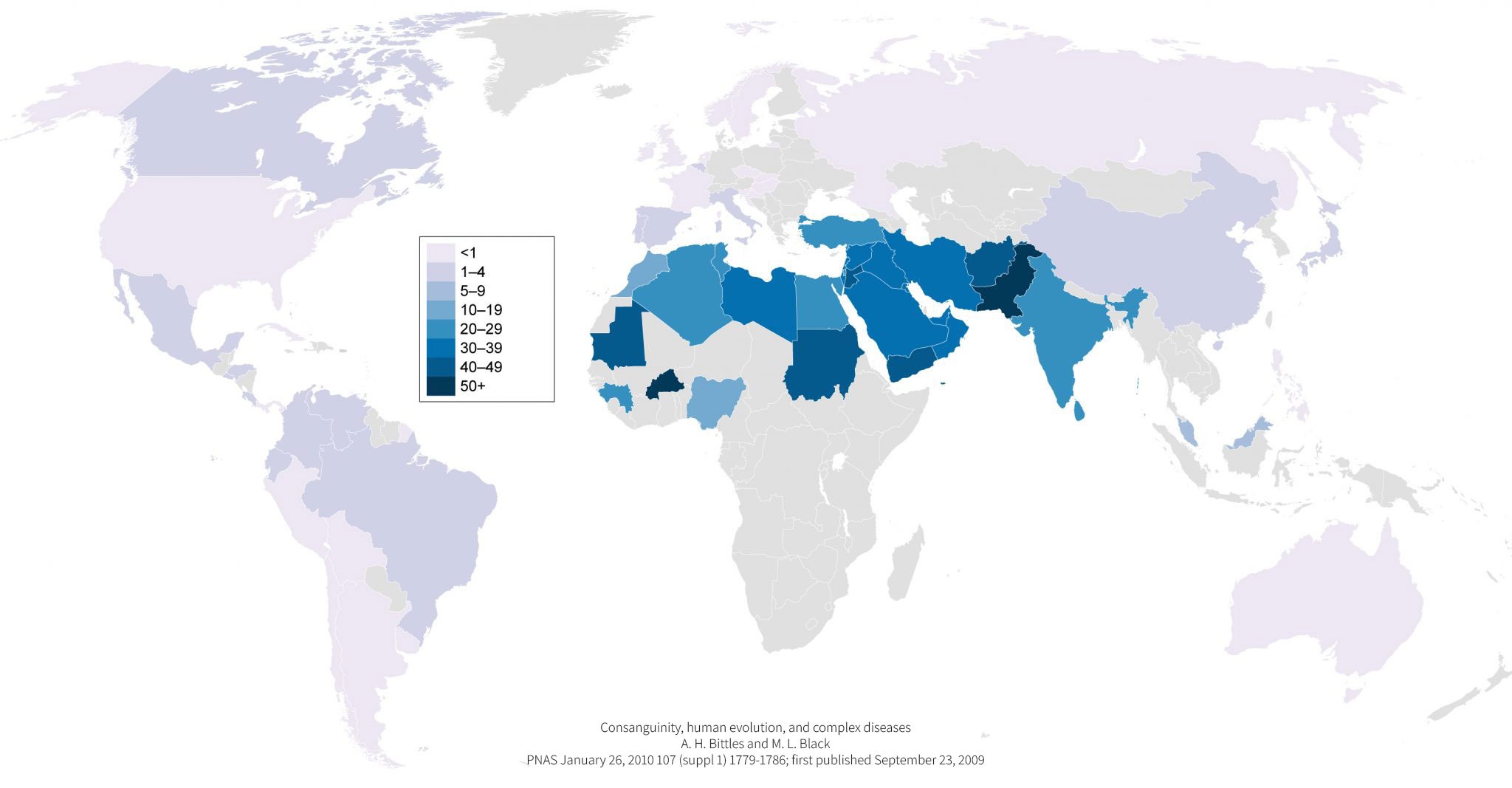
Rare diseases are, by definition, seldom, and hard to diagnose. Companies, as yours, willing to run clinical trials often face problems in recruiting enough eligible patients for their phases II & III clinical studies.
CarthaGenetics® will accelerate your recruitment for such patients through its strong relationships with KOLs, local scientific societies and local patient groups.
With more than 20 years experience in genetics disorders, CarthaGenetics® has developed a very strong expertise in inborn errors of metabolism which have higher incidences in South Mediterranean countries where consanguinity levels are much higher.
CarthaGenetics® will collaborate with your set up your different clinical trials site in a strategic way. We will also deeply collaborate with CRO to accelerate patients identification, clinical trial approvals, screening and potential inclusion.
We are also helping in transferring patients to other country if there is no possibility to open a local site for ultra rare disease and/or gene therapy.
Please ask for other examples and references at: info@carthagenetics.com
Rare diseases are, by definition, seldom, and hard to diagnose. Companies, as yours, willing to run clinical trials often face problems in recruiting enough eligible patients for their phases II & III clinical studies.
CarthaGenetics® will accelerate your recruitment for such patients through its strong relationships with KOLs, local scientific societies and local patient groups.
With more than 20 years experience in genetics disorders, CarthaGenetics® has developed a very strong expertise in inborn errors of metabolism which have higher incidences in South Mediterranean countries where consanguinity levels are much higher.
CarthaGenetics® will collaborate with your set up your different clinical trials site in a strategic way. We will also deeply collaborate with CRO to accelerate patients identification, clinical trial approvals, screening and potential inclusion.
We are also helping in transferring patients to other country if there is no possibility to open a local site for ultra rare disease and/or gene therapy.
Please ask for other examples and references at: info@carthagenetics.com
