- HOME
- »
- QUESTIONS ABOUT EAP
IS YOUR PRODUCT ELIGIBLE FOR EAP ?
To be eligible for an EAP, your drug candidate must fulfill several criteria
♦ Regulations state that a treatment can be available to patients under EAP who have a chronically or seriously debilitating disease, or a life-threatening disease, and who cannot be treated satisfactorily by an authorized medicinal product.” These programs are not only designed for rare diseases but also for severe conditions affecting large populations.
♦ Your drug candidate must either be the subject of an application for marketing authorization or must be in late stage clinical trials. Typically, EAPs involve products in Phase III (in a few cases, just after Phase II). Sufficient safety and efficacy information are required to demonstrate a positive benefit/risk ratio and permit use of the drug in a minimally controlled out-patient setting.
♦ EAP shall not interfere with or replace your clinical trials. Although safety data may be collected during EAPs, these programs are not the substitutes for clinical trials. Therefore, patients should always be considered for your on-going clinical trials before being offered a drug candidate through an EAP.
For detailed information, please contact us at: eap@carthagenetics.com

WHEN TO PLAN AN EARLY ACCESS PROGRAM ?
When to plan an EAP ?
Planning for a managed access program should be considered as early as possible from Phase II trials onwards. This allows time for preparation of standard operating procedures, consultation with regulatory authorities for approval, agreeing on the label and development of information for physicians and pharmacists regarding dosing, administration and restrictions.
For more information on countries allowing NPS, pricing, reimbursement, patient inclusion criteria, supply, required resources and processes, please contact us at: eap@carthagenetics.com
WHEN TO PLAN
AN EARLY ACCESS PROGRAM ?
When to plan an EAP ?
Planning for a managed access program should be considered as early as possible from Phase II trials onwards. This allows time for preparation of standard operating procedures, consultation with regulatory authorities for approval, agreeing on the label and development of information for physicians and pharmacists regarding dosing, administration and restrictions.
For more information on countries allowing NPS, pricing, reimbursement, patient inclusion criteria, supply, required resources and processes, please contact us at: eap@carthagenetics.com
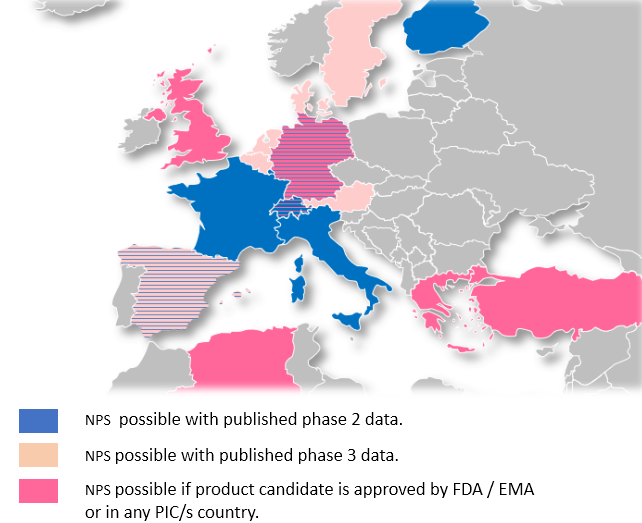
WHICH COUNTRIES ARE ALLOWING NAMED PATIENTS SALES ?
Due to difference in legislation, EAPs to Orphan Drugs vary much across Europe. The following map shows, where NPS/EAP are possible in principle. You will note that countries allowing EAP from Phase II represent a total population of more than 210M inhabitants.
To start your Early Access Program in countries of your choice, please contact us at: eap@carthagenetics.com
WHICH COUNTRIES ARE ALLOWING
NAMED PATIENTS SALES ?
Due to difference in legislation, EAPs to Orphan Drugs vary much across Europe. The following map shows, where NPS/EAP are possible in principle. You will note that countries allowing EAP from Phase II represent a total population of more than 210M inhabitants.
To start your Early Access Program in countries of your choice, please contact us at: eap@carthagenetics.com

WHO WILL PAY FOR YOUR PRODUCT DURING NPS/EAP PHASE ?
Usually, hospitals and/or national insurance, or insurances systems bear the costs of Named Patient Sales in most countries (France, Italy, Turkey, Switzerland and Spain).
It is important to consider that if your drug is charged, your EAP price might be used as a benchmark for pricing and reimbursement committees in Europe.
For more info, please contact us: eap@carthagenetics.com
WHO WILL PAY FOR YOUR PRODUCT DURING NPS/EAP PHASE ?
Usually, hospitals and/or national insurance, or insurances systems bear the costs of Named Patient Sales in most countries (France, Italy, Turkey, Switzerland and Spain).
It is important to consider that if your drug is charged, your EAP price might be used as a benchmark for pricing and reimbursement committees in Europe.
For more info, please contact us: eap@carthagenetics.com
WORKING WITH CARTHAGENETICS ON YOUR EAP/NPS
Implementation and management of an EAP requires significant knowledge, experience and resources. Your company may not have all the specific experience in this highly regulated environment nor the dedicated resources.
CarthaGenetics® is your partner of choice in setting up EAP for you with local people who have broad experience and well-established professional relationship with KOLs, local health authorities, scientific societies as well as patient groups and KOLS.
♦EAPs require managing small quantities in a number of different European markets. This approach is completely different than the traditional marketing methods (like detailing and informing physicians via traditional sales calls or mailings), which would be both inappropriate and prohibited by law for EAPs, as non-registered products cannot benefit from active promotion, whatever the country. CarthaGenetics® team is experienced in operating within the local regulatory guidelines.
♦ CarthaGenetics®’ approach is always ethical and the team is totally dedicated to help suffering patients. CarthaGenetics® aims to identify national champions who will help the patients in urgent need have access to an early treatment.
♦ Setting up an EAP requires a lot of paperwork like individual applications, approval processes, renewals of those orders, compliance follow-up. Physicians and pharmacists have to start the procedure for each patient and follow-up with individual applications. Carthagenetics® team is trained to give support locally in accordance with the regulations.
♦ CarthaGenetics® helps with all distribution activities, including setting up direct delivery, training of caregivers, pharmacists and patients, complaint handling and adverse event reports to your pharmacovigilance, where needed/applicable.
CarthaGenetics® has the expertise, experience, SOPs in place that will provide absolute peace of mind that the program is devised and managed effectively and within local regulatory guidelines. With our proven success track, your EA program will be robustly implemented taking into consideration national regulatory nuances.
For detailed information, please contact us at: eap@carthagenetics.com
